
Apex® EZTest®: A Self-Contained Biological Indicator for Vaporized Hydrogen Peroxide Decontamination Processes
Mesa Laboratories is proud to announce the release of a new product, Apex EZTest, a self-contained biological indicator (SCBI) for monitoring the efficacy of ambient pressure vaporized hydrogen peroxide (VH2O2) processes (Figure 1).
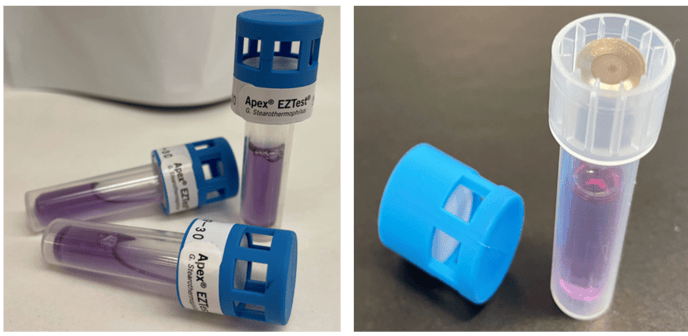 Figure 1. Apex EZTest Biological Indicator, (right image displays spore disc placement)
Figure 1. Apex EZTest Biological Indicator, (right image displays spore disc placement)
This unique patented SCBI design keeps the spore carrier positioned directly underneath the Tyvek® filter in the cap allowing easy VH2O2 access to the spores. Apex EZTest is user-friendly and eliminates the need for post-treatment transfer to a laboratory for spore cultivation. Each SCBI unit contains the culture vial and growth medium as onboard components allowing aseptic cultivation to occur in any setting. This design essentially eliminates the occurrence of post-processing contamination, which is a source of false-positive BI results.
Apex EZTest rounds out Mesa’s robust Apex product line that offers quality biological indicators customers can rely on to monitor or validate their VH2O2 cycles in isolators, restricted access barrier systems (RABS), and other enclosures. Apex EZTest was designed primarily for organizations involved in the aseptic manufacture of pharmaceutical and medical device products. The product is also suitable for laboratories and other enclosed facilities that require periodic decontamination.
Apex EZTest SCBIs consist of a 9 mm diameter x 0.2 mm thick stainless-steel disc inoculated with ≥1.0 x 106 Geobacillus stearothermophilus spores (strain 12980), which is placed into a thermoplastic vial that serves as a culture tube. A small glass ampoule containing sterile culture medium and pH indicator is also contained in the vial. A Tyvek filter, which is permeable to VH2O2, is placed over the top of the culture tube to prevent contamination, and a cap with large vents is set over the top which allows VH2O2 to access the inoculated disc during exposure.
The Apex EZTest SCBI has a shelf-life of 9 months when stored at 2-8℃ and <50% relative humidity. Apex EZTest units are packaged in resealable pouches (Figure 2). Between use, it is recommended that the desiccant is not removed, and the pouch remains sealed.

Figure 2. Apex EZTest BI with sealed pouch
The culture medium, consisting of a proprietary formulated soybean casein digest base, is filled and hermetically sealed into glass ampoules. Following manufacture, the ampoules are autoclaved to render them sterile and growth promotion is performed using less than 100 spores of G. stearothermophilus. The sealed ampoules are a defined size and are placed into the plastic body with the inoculated disc. The ampoule is an ‘onion skin’ glass that allows it to be easily crushed when the plastic body is compressed. This provides the spores with a nutrient medium for growth.
The culture medium contains a pH indicator (Bromocresol purple), which is purple in color in an unactivated unit. After activation (when the plastic body is compressed and the disc is submerged into the media) and an appropriate incubation period, spore grow-out is indicated by a color change from purple to yellow. If the medium remains purple, the spores did not grow, indicating they were killed in the decontamination process. Therefore, if the decontamination process was not effective, the spores will grow and turn the medium cloudy and/or yellow (Figure 3). The Apex EZTest is labeled for a 7-day incubation; however, positive results are often observed earlier in the incubation period. If any ampoules show signs of a visual color change, or turbidity, prior to use, they should be autoclaved and discarded.
 Figure 3. Apex EZTest BI (left unit is unactivated while the right unit is growth positive after incubation)
Figure 3. Apex EZTest BI (left unit is unactivated while the right unit is growth positive after incubation)
Apex EZTest is designed for use during the development, validation, and re-validation of VH2O2 decontamination processes. They can also be used to monitor the effectiveness of routinely performed decontamination cycles.
Apex EZTest SCBIs can be conveniently placed throughout the exposure environment either standing upright on their own or hung from a hook so that all areas of interest in the enclosure can be evaluated (Figure 4).
.png?width=688&name=Untitled%20design%20(37).png)
Figure 4. Apex EZTest BI placement (either standing on a surface or hanging from a fixture)
The Apex EZTest SCBI offers a unique advantage because the SCBIs can be activated (cultured) outside of an aseptic environment, eliminating the added time and resources required for culturing standard disc BIs. This is accomplished simply by compressing the sides of the vial to a point where the media ampoule ruptures. This act not only releases the growth medium but also frees the spore disc from its fixed position under the filter. As the growth medium is inherent in the SCBI, no other source of tubed media is required.
The Apex EZTest employs the same basic features as a standard disc BI used in VH2O2 processes. In both product formats, the inoculated stainless-steel disc is packaged facing the permeable Tyvek barrier, allowing the VH2O2 to interact with the spores. The previously discussed post-treatment cultivation of the SCBI is where the user will see the greatest benefit over the traditional spore disc BI. The resistance of the EZTest is assessed in Mesa’s test isolator (2 mg/L VH2O2). The D-value is determined using the Fraction Negative method and calculated using the Stumbo-Murphy-Cochran procedure.
The Apex EZTest can be activated either by hand or by its accessory device, the Apex EZTest Crusher (Figure 5). The Apex EZTest unit is easily activated in three distinct steps: break the media ampoule, crush the glass until the disc is completely submerged within the culture medium, and then depress the cap until a “CLICK” is heard and felt. The Apex EZTest Crusher was designed to ergonomically assist in activating each unit and up to three units can be simultaneously closed (capped). The EZTest Crusher can withstand exposure to VH2O2 in the instance that the end-user would like to use it as a holder for the units during decontamination.
.png?width=690&name=Untitled%20design%20(38).png) Figure 5. The Apex EZTest Crusher accessory
Figure 5. The Apex EZTest Crusher accessory
The Apex EZTest SCBI is a welcome addition to the family of Apex biological indicators and a summary of its positive features includes:
- Eliminating the need for a Clean Bench during culturing activities
- Minimizing post-process contamination
- Reducing inventory since no additional media is needed for culturing
- Providing easy-to-interpret results from a visible color change when growth positive
- Exhibiting positive results frequently before full 7 days of incubation.
Additional information on this product as well as BI use in the development and validation of a decontamination process can be found on the Mesa website.
References
- Spore News SN043 Parameters Affecting Vapor Hydrogen Peroxide BI Performance
- Spore News SN044 Using Replicate BIs to Evaluate Biodecontamination Cycles in Isolators
- Spore News SN045 End User—Proper BI Placement During Vaporized Hydrogen Peroxide Decontamination Cycles
- Spore News SN051 SEM Analysis of Vaporized Hydrogen Peroxide Biological Indicators
- Spore News SN072 Vaporized Hydrogen Peroxide Isolator Decontamination in a World of Uncertainty: A Modern-Day Field Guide
- Spore News SN073 Biological Indicators for Vaporized Hydrogen Peroxide Isolator Decontamination: Characteristics, Uses and Challenges
- PIC/S, Pharmaceutical Inspection Convention Co-Operation Scheme, “Recommendation on the Validation of Aseptic Processes”, 01 Jan 2011.
- US Pharmacopoeia, General Information, Chapter <1208>, Sterility Testing—Validation of Isolator Systems, Guidance on isolator design and construction.
SN074-V1