Accelerating Innovation, Caring for Communities
Learn how we’re Protecting the Vulnerable® in the second edition of our Environmental, Social, and Governance Program Brochure.
Making compliance possible
From precision-engineered solutions for unique applications to advanced technologies and breakthrough features, Mesa Labs helps you meet the most demanding quality standards. Our comprehensive portfolio of equipment, software, systems, and services makes it easy to meet – or exceed – any requirements.

“When it comes to quality control, there’s always a way to improve. We believe in our solutions, and we stand behind them.”
Our growing list of certifications and approvals helps you meet the most demanding standards.
| EPA | 21 CFR Part 11 |
| FDA | CSA International |
| ISO/IEC 13485:2016 Certified | Accredited to ISO/IEC 17025:2017 |
Certified customer support
Find answers to your questions and get the help you need.
Calibration & Service
Stay compliant, get authorized service, and troubleshoot with the experts.
Resources
Access specs, videos, FAQ, and more in our document library
Consulting
Put experience on your team from design to deployment.
Shaping the future
Insights, news, and best practices to keep you on the leading edge of quality and compliance.

OUR MISSION
Protecting the vulnerable®
Mesa Labs sets priorities according to customer needs and in service of our purpose: pushing technological boundaries in order to protect the vulnerable.

DOWNLOADS
Resource Library
Find your product and solution documentation, all in one convenient location.
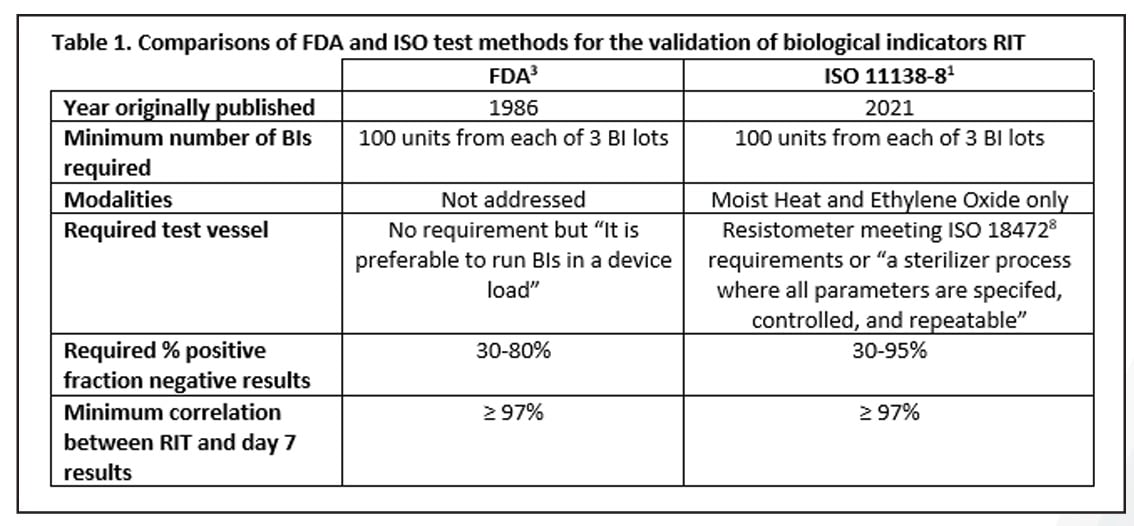
STANDARDS & COMPLIANCE
A Review of the Newly Released ISO 11138-8 Standard on the Validation of a Reduced Incubation Time for Biological Indicators
Read Mesa Labs' Spore News to learn about the newly developed ISO standard for the validation of a reduced incubation time as part of the 11138 series.

SOLUTIONS
The MeCo Solution: Improving Consistency, Accuracy and Robustness in SIP Validation
Read Mesa Labs’ Spore News to learn about how MeCo improves consistency, accuracy and robustness in sterilization in place (SIP) validation.
SERVICES
Contact us for expert support
Reach out to us today by filling out our online form.