VOL 11, ISSUE 4
Delayed Incubation, Part II: The DriAmp Product
By Eric Gillitzer, Ph.D.
Previously, we discussed delayed incubation with regards to steam sterilized Self-Contained BI EZTest product (Spore News Vol. 10, No 4). Here, we address delayed incubation with a very different BI used in a different process: DriAmp for Dry Heat sterilization.
In the ‘Resistance Performance Tests’ section of the USP, it states to culture a biological indicator (BI) ‘‘within a noted time, not more than four hours’’ after the sterilization process1. An issue arises where the end user is unable to culture the exposed BI within the 4-hour window. As alluded to previously, in those cases, is a delay of a day, two or a week acceptable between the time of exposure and culturing of the BI?
Two studies were performed by Mesa Labs, Bozeman Manufacturing Facility (BMF) with the DriAmp product for dry heat sterilization. In these studies, D-values were established with BIs cultured immediately after exposure and compared to D-values from BIs that had been held after exposure. To establish an acceptable window for comparison, the D-value of the delayed incubation BI samples needed to remain within 20% of the original assay D-value. This is not the exact intent of the ISO standard for confirming D-value as indicated in ISO 11138-12, but in assaying the ability of injured spores to remain viable within a similar 20% window an acceptable measure would be established. The lots used for these studies are indicated as Lot “A’, Lot “B’ and Lot “C’. Units from Lots “A’ and “B’ were exposed, held, shipped and cultured separately from units of Lot “C’.
[1] USP 31, <55>, Biological Indicators- Resistance Performance Tests, Recovery
[2] ISO 11138-1 Sterilization of Health Care Products, Biological Indicators Part 1
For the first study examining the effects of shipping exposed BIs, units from three lots of BIs were exposed in our dry heat Biological Indicator Evaluator Resistometer (BIER) at 160 °C such that fractional results were obtained. Each group of 30 units was split into three-10 unit sets. One set was cultured and incubated at 37 ± 1 °C for 72 hours and scored for growth. One set was held at room temperature at the Mesa Labs, BMF facility and the remaining set of units was shipped to and returned from a distributor in France. Approximately 8 days were required for transit to and back from France. Upon return, the held and shipped units were cultured and incubated as above and scored for growth at 72 hours. As a reference, a temperature data logger was placed with the stored, exposed units and a data logger was included with the exposed BIs shipped to France and back.
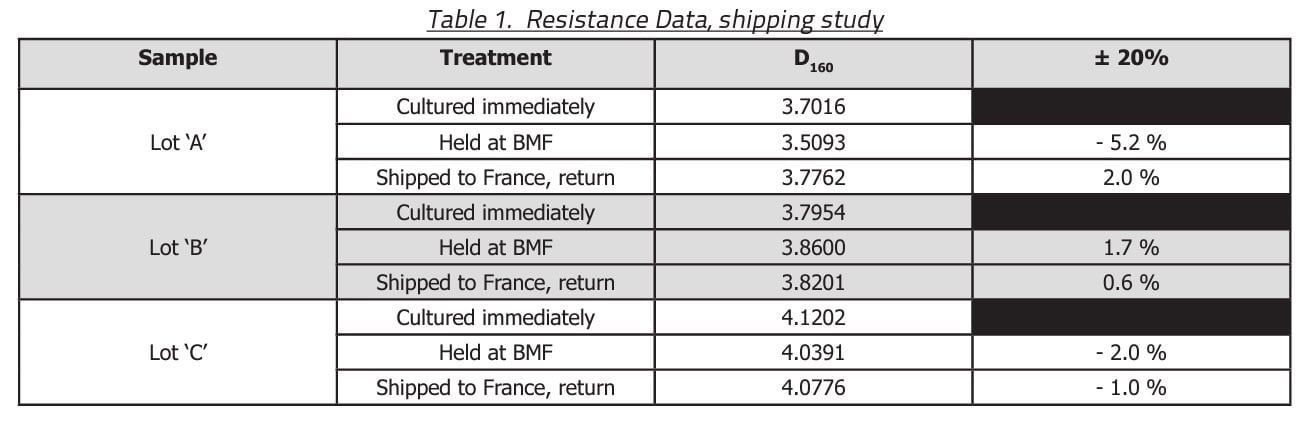
For those units held at BMF, the temperature varied between 19-25 °C and the relative humidity varied between 24-52 % during the duration of the study. For those units that had travelled to France and back, the temperature varied between 4-33 °C and the relative humidity varied between 21-65 %. Shown in Table 1 are the D160 values for the three lots of BIs before and after the duration of the shipping study.
For the second study examining delayed incubation without shipment, DriAmp BIs were again exposed in the dry heat BIER at 160 °C such that fractional results were obtained. Each group of 40 units was split into four-10 unit sets. One set was cultured and incubated immediately at 37 ± 1 °C for 72 hours and scored for growth. The remaining sets of units were stored at room temperature at the Mesa Labs, BMF facility for 1-7 days post exposure. Following the delay, BIs were cultured and incubated as previously described. Temperature monitoring was performed with a data logger placed in the immediate area where the exposed units were stored.
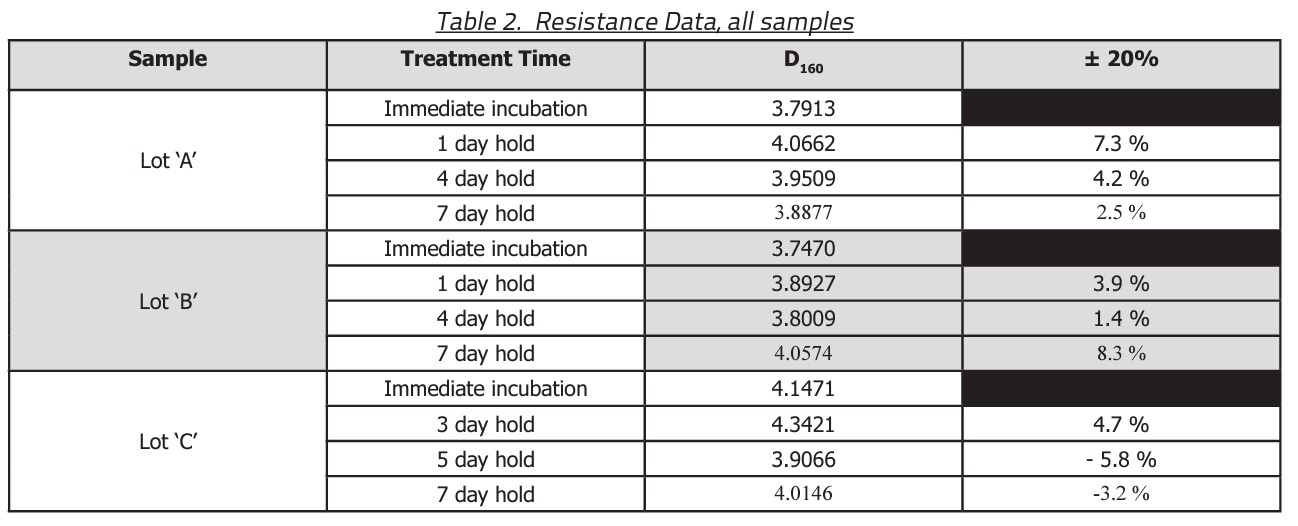
Temperatures during storage ranged between 20-25 °C. Relative humidity during the study was varied between 21-52 %. Shown in Table 2 are the D160 values for the three lots of BIs and hold time prior to incubation.
In all, these studies seem to indicate that holding and shipping DriAmp BIs for up to 7 days, or possibly longer, after exposure has little deleterious effect on the survivability of spores present in the BI.
About the Author
Eric Gillitzer, Ph.D., is a Senior Research Scientist for Mesa Labs. He started in 2008 in the production area and since then has been involved in all aspects of BI manufacture and testing in the production lab, spore lab and R&D laboratories.
Eric is a member of the Association for the Advancement of Medical Instrumentation (AAMI) and a former member of the American Society for Virology (ASV) and American Society for Microbiology (ASM). Eric graduated from Montana State University, Bozeman with a B.S. in Microbiology and from SUNY Stony Brook with a Ph.D. in Molecular Biology and Biochemistry.