
What is the D-ifference in D-value?

Occasionally our Customer Service Department receives a request for biological indicators (BIs) with either a very specific and/or narrow range of acceptable D-values. As an example, a recent order was received in which the customers’ specification for EZTest® Gas BIs was a D-value not to exceed 3.5 minutes. Scanning the available inventory, it was determined that a lot with DEO-value of 3.6 minutes was available for immediate shipment. (In fact, the non-rounded D-value for this lot was 3.5556 minutes.) The client declined to receive that lot and requested that a new lot be manufactured to meet the 3.5-minute criteria.
SQUARE WAVE LETHALITY
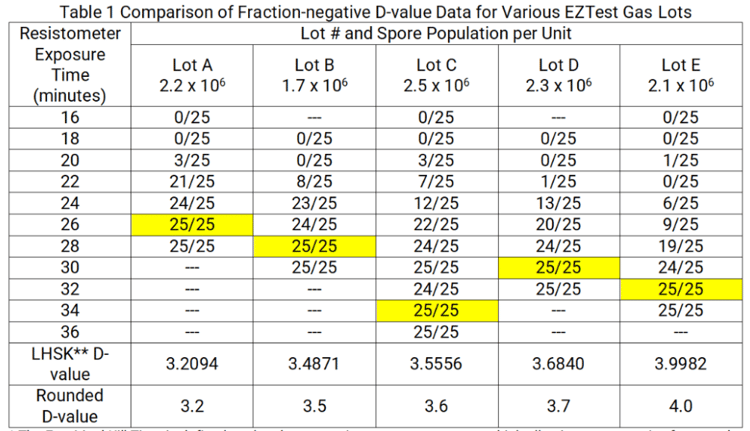
To determine whether a BI with 3.5-minute DEO-value is acceptable for use in a process cycle, while any value in excess of 3.5 minutes is unacceptable, consider the fraction-negative EZTest Gas D-value data presented in Table 1. The values are presented as the number of negative units/number of units exposed with Empirical Kill Times* highlighted. At a quick glance, the data sets in Table 1 appear different, but are they really different?

These subtle differences in fraction-negative data are detectable only in a resistometer that delivers “square-wave” lethality. The term square-wave is applicable in that a resistometer controls within a very tight set of exposure conditions and during the exposure dwell phase, steady-state conditions are achieved and maintained. A resistometer will1:
1. Charge with EO gas from 0 to 600 mg/L in 60 seconds
2. Maintain gas concentration to within 5% of target mg/L
3. Control exposure temperature to within ± 1ºC
4. Maintain relative humidity to within ± 10% of target
5. Evacuate the chamber to 10 kPa or less within 60 seconds
As a result of this type of cycle performance, all the lethality in each resistometer cycle is delivered to the BIs in one concise and succinct dose.
By comparison, a process vessel operates with much more variation in conditions. ISO 11135:2014, Annex D states that a temperature range for an empty chamber during EO or inert gas exposure of +/- 3ºC of the set point is permissible; however, when loaded, the ±3°C tolerance might not be achievable.
Based on the variation in critical process parameters that exists within a loaded production sterilizer, steady-state conditions may never exist. During an EO production cycle:
1. Residual air molecules are attempting to exit the product and packaging material
2. Ethylene oxide and nitrogen gas molecules are attempting to enter the product and packaging material
3. Heat penetration continues and the cooler product locations experience a rise in temperature
4. As product temperature increases, water molecules are driven out of the product, thus reducing relative humidity at that location
5. Water molecules are also being absorbed by packaging material in competition with the product
A vast array of molecules is attempting to reach a state of equilibrium within the loaded vessel. The temperature of the product influences molecular activity while pressure changes in the vessel have an effect on the direction of molecular movement.
The critical parameters are dynamic and constantly fluctuating such that chaotic-state conditions would be a more accurate description of the lethality delivered in a process vessel. This molecular chaos takes a long time to reach equilibrium and may never achieve such a condition in typical cycle times. Unless the temperature stabilizes, molecular equilibrium will not occur. The spores respond to the exact conditions prevailing at the specific location of the BI. Unlike a probe that records only one process parameter in one location within the chamber, the spores integrate the overall lethality that exists throughout the product load as a result of these dynamic process conditions.
PROCESS VESSEL LETHALITY
Whereas the tight performance parameters of a resistometer result in delivery of square-wave lethality, the broad range of performance in a production sterilizer results in “process vessel lethality.” In contrast to the 60-second charge time in a resistometer, the gas charge phase to reach the appropriate concentration in a process vessel may take 10 to 40 minutes. Subsequent attainment of exposure temperature within the load may take even longer; during which time, lethality is being delivered to the bacterial spores in the BIs despite the fact that we have yet to achieve the exposure process parameter set points. The exhaust time in a process vessel may take a long time depending on the product packaging. Due to the comparatively long times to attain exposure conditions and subsequent removal of EO gas as well as the chaotic-state conditions that prevail during the exposure phase in a process vessel, slight differences in BI resistance performance, such as those seen in Table 1, cannot be detected in a process vessel.† The accumulated lethality delivered during only the gas charge and exhaust phases is often sufficient to inactivate the spore challenge of the BI. This was demonstrated in a study by Mosley et al (2002)2 in which zero-minute exposures were run for validation testing of various medical products. Using both spore strips and self-contained BIs, the accumulated lethality delivered in the zero-minute exposures resulted in few or zero positive test units.1

What then is the function of a BI if most of the test units will be inactivated by the lethality delivered only in the gas charge and exhaust phases of a process cycle?
When BIs are used in validation the approach is either 1) a combination BI/bioburden approach or 2) an overkill approach. Most of the customers we work with use the overkill approach, the most popular of which is the half-cycle method.3
Overkill validation by design has a significantly extended process beyond where the BIs were demonstrated to be killed. This extra process time is equated to Sterility Assurance Level (SAL). Therefore, if a BI is positive (survives a validated cycle), then all the “extra” lethality designed into the process has been “lost”.
The BI as a routine monitor is therefore a system to be used to detect catastrophic process failures; such failures do occur. Currently, no physical instrumentation systems are available that can be embedded in the most difficult to sterilize location of the product to measure all critical process parameters. As previously stated, the bacterial spores in the BI effectively integrate all critical process parameters at all times during the cycle and respond to the exact lethal conditions that exist in the specific location in which the BI is placed.
An example of a catastrophic failure is inadequate moisture (humidity) where the BI challenge is located in the load. When humidity is too low, 90% or more of the expected lethality of the process can be lost. The “unexpected” positive test unit in a validated process is not due to the use of a BI whose D-value is slightly greater than the desired level, it is due to the loss of a critical process condition(s) and loss of a significant portion of the microbial lethality.
While the D-value may have an effect during cycle development and validation testing, slight lot-to-lot differences in D-value (detectable in a resistometer) will not result in positive BIs when used in a full-cycle production vessel.
THE DIFFERENCE IN D-VALUE
As outlined above, D-value assessment is conducted in a resistometer with tightly controlled cycle parameters. ISO 11138-1 states that when a manufacturer confirms the D- value reported on the Certificate of Analysis (CoA), results within +/- 20% of the label claim are considered adequate reproduction of the original assessment. Thus, even with the precise performance capabilities offered by a resistometer, it is recognized that small differences in retest data are expected. From Table 1, the median D-value is 3.6 minutes. Using the +/- 20% criteria, retest values that were within 2.88 to 4.32 minutes would be acceptable reproductions of the 3.6-minute label claim. From a resistance performance perspective, the lots presented in Table 1 could be considered identical as all D-values are within +/- 11% of the median 3.6-minute value.
Regardless, we do encounter customers who decline to use a BI with a D-value even slightly higher than their requested maximum value for fear of observing a positive test result. In a recent communication, an EZTest lot with a D-value of 3.5556 minutes was considered not acceptable. Upon review of the resistance performance data, the customer was concerned that the high population per unit (2.5 X 106) in conjunction with the D- value (3.6 minutes, as reported on the CoA) would result in a calculated kill time that is too robust ††. In this instance, the D-value exceeded the clients’ 3.5-minute maximum specification by 0.0556 minutes (3.3 seconds)*.
*In the fraction negative data set for the 3.5556-minute D-value lot, 225 BIs were used to span the empirical survival time to the empirical kill time range. Of those 225 BIs, 142 tested negative for growth. Had one fewer BI tested positive (i.e., 143 negative replicates of the 225 exposed) the non-rounded D-value would be 3.5436 and the rounded value reported on the CofA would have been 3.5 minutes.

CALCULATED VS EMPIRICAL SURVIVAL AND KILL TIMES
The Calculated Kill Time is derived using the equation:
Calculated Kill Time (minutes) = (Log10 population + 4) X (D-value)
The Calculated Survival Time is derived using the equation:
Calculated Survival Time (minutes) = (Log10 population - 2) X (D-value)
While USP and ISO require the manufacturer to include the calculated survival and kill times on the CoA, these numbers are of limited value when viewed in terms of process spore log reduction (SLR). To illustrate this aspect, consider the resistance performance for EZTest Gas Lot B (see Table 1). With D-value defined as the time at a specified set of exposure conditions necessary to reduce the viable spore population by one log, the following death kinetics are applicable.
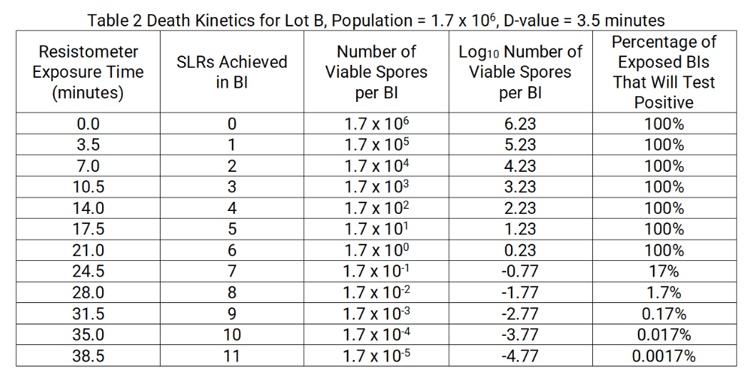
Notice from Table 1 that when conducting D-value assessment, the empirical survival time and empirical kill time for Lot B were observed to be 20.0 and 28.0 minutes, respectively. Table 2 predicts an empirical survival time of 21.0 minutes (not tested during D-value assessment) and an empirical kill time of 28.0 minutes.†
† 25 BIs exposed in each cycle
1.7 % of test units will be positive
25 X 0.017 = 0.425 positive test units
since 0.425 < 1, zero positive of the 25 exposed is expected
With 1.7 X 106 population and 3.5-minute D-value, the calculated survival time for Lot B is 14.81 minutes and the calculated kill time is 35.81 minutes. Recall that the empirical kill time for Lot B is only 28.0 minutes, a difference of 7.81 minutes.
The reason for the discrepancy between the empirical kill and the calculated kill time is the “+4” that appears in the calculated kill time equation. As can be seen in Table 2, when starting with 106 spores per BI, eight SLRs (i.e., two SLRs in excess of the starting population) are needed to achieve no growth among the exposed test units. The “+4” in the calculated kill time equation results in an exposure time that will deliver ten SLRs to the 106 starting population. This level of lethality is well in excess of what is necessary to inactivate the spore challenge.
As stated previously, the client rejected the 3.6-minute D-value (Lot C) stating that the calculated kill time would be too great. The calculated kill time for the 3.5-minute D- value (Lot B) is 35.81 minutes and this lot was considered acceptable to the client.
Notice from Table 1 that if any of the lots were exposed in a 35.81-minute resistometer exposure, ALL test units would be inactivated, including those with a D-value of 4.0 minutes.
CONCLUSION
EO resistometer standards require the specified set of conditions to be maintained at 600+/- 30 mg/L EO, 54 +/- 1ºC, 60 +/- 10% RH. Also required is the attainment of EO gas concentration and subsequent evacuation of the chamber to be achieved in 60 seconds or less. Such exact conditions result in the attainment of steady-state conditions and delivery of square-wave lethality which allows one to observe the slight differences seen in Table 1. However, when exposed in a process vessel it is impossible to ascertain these subtle differences in resistance performance.
We must be careful to not implement specific performance requirements where such limiting factors are not warranted. The perception that a BI must have a specific and narrow performance range for monitoring validated process cycles is an inappropriate approach.
The unexpected positive test unit in a validated process is not due to use of a BI with a D-value slightly greater than the desired level. The positive BI results from the loss of critical process conditions and the loss of a substantial portion of the microbial lethality. The D-value on the Certificate of Analysis is an accurate estimate of resistance for a specific set of exposure conditions. It can have an effect during cycle development but is not a concern for positive BIs in a normal, full-cycle production run.
REFERENCE
1. ISO 18472:2018 Sterilization of health care products – Biological and chemical indicators – Test equipment
2. GA Mosley, JR Gillis, JE Whitbourne, “Calculating Equivalent Time for Use in Determining the Lethality of EtO Sterilization Processes,” Medical Device & Diagnostic Industry, February 2002.
3. Spore News SN080 How to Prove a Sterility Assurance Level of 10-6 PART 1: The OVERKILL Method(s)
SN012-V1