
Product D-Value Studies: a Critical Tool When Developing a Sterilization Process
By Kurt McCauley
The objective of a sterilization process is to kill the naturally occurring microbial contamination (bioburden) present in the product. Two common sterilization methods used are the bioburden (BB) method and the overkill method. The BB method is defined as a sterilization process with parameters based on the predetermined type and concentration of bioburden on the material being sterilized. The overkill method is defined as a sterilization process that is based upon an arbitrarily established higher initial concentration and resistance of bioburden than that actually expected on the material being sterilized.
1. What is the bioburden of the product?
The microorganisms present in a product can be viral, bacterial, and fungal and come from three main sources: the manufacturing environment, personnel, and raw materials including water. Some organisms are spore formers, which generally are the most resistant to environmental stresses including sterilization processes. The product may have little or no bioburden depending on the manufacturing conditions and raw materials, or it may contain high concentrations of multiple organisms. It is essential to learn as much as possible about the bioburden organisms, especially when running minimal sterilization cycles (due to product sensitivity to the sterilization process). Information required when characterizing the bioburden include:
- Total numbers of organisms present just prior to sterilization
- Identity of organisms present
- Number of spore formers present
- Resistance of the bioburden
- Sampling frequency and statistical analysis
2. What effect does the product have on the bioburden prior to sterilization?
It is important to know how the product will influence the behavior of the organisms present. This is particularly important in liquid products. If the product is capable of promoting growth, then over time, a few organisms could eventually result in high numbers of organisms. This presents a problem if there is a significant time delay between manufacture and sterilization. High numbers of organisms could result in an increase in sterilization time. Alternately, the material may be bacteriostatic, bactericidal, sporostatic, or sporicidal.
Properties of the material being sterilized (e.g., the chemistry of a pharmaceutical product or the physical characteristics of a medical device) can have a significant influence on the resistance of the microorganisms. The product may protect the organisms in numerous ways such as by coating them or causing them to clump, increasing their resistance to the sterilization process. Alternatively, it may make them more susceptible to the sterilization process.
Figure 1 displays single free floating spores, while Figure 2 displays several large clumps, some of them containing hundreds of spores.
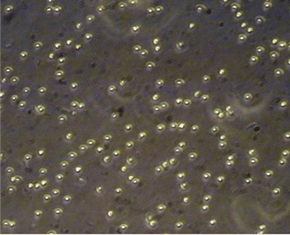
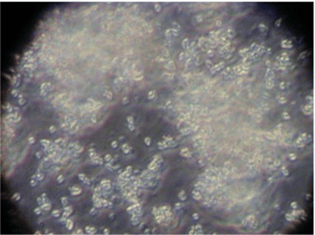
Figure 1. Non-clumped Spores in Ethanol Figure 2. Clumped Spores in Product
3. Why is it acceptable to base sterilization cycles on the resistance of Geobacillus stearothermophilus spores?
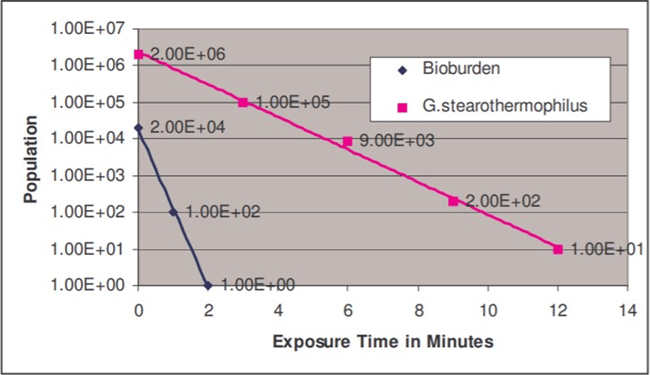
Geobacillus stearothermophilus (ATCC #7953) spores (which typically have D121-values in the 1.5 - 2.5 minute range on a paper strip) are more resistant than most wild-type organisms. These spores, widely used for monitoring steam sterilization processes, may be substituted for the bioburden organism during resistance testing. When this substitution occurs, it is assumed that the G. stearothermophilus spores are more resistant than the bioburden organisms or by design significantly outnumber the bioburden organisms. The kill time for the G. stearothermophilus would then exceed the kill time for the bioburden organisms. The following chart illustrates this point.
4. Is it acceptable to base sterilization cycles on the resistance of other organisms?
Other organisms such as Bacillus coagulans (ATCC #7050), B. smithii (ATCC #51232), B. subtilis “5230” (ATCC #35021) and Clostridium sporogenes (ATCC #7955) may be used in certain situations. These organisms are more sensitive to moist heat sterilization than G. stearothermophilus, but in general, they are still more resistant than the bioburden. It may be desirable to use one of these organisms when the product is sensitive to the sterilization process, thereby limiting the sterilization time. However, when working with a heat-sensitive product, it is strongly recommended that the bioburden be characterized prior to basing sterilization parameters on the above-mentioned indicator organisms.
5. Why perform a D-value study?
The most direct method for evaluating the resistance of the test organism (a bioburden isolate or G. stearothermophilus spores) is by performing a D-value study. When evaluating the bioburden, it is not necessary to test every organism isolated, only the most resistant (which can be isolated through the use of a heat shock screening procedure).
6. How are D-value studies performed?
Ideally, the D-value study should be performed on the actual product spiked with the test organism. Resistometers1, formerly Biological Indicator Evaluator Resistometer (B.I.E.R.) vessels, capable of running square wave cycles, are commonly used in D-value studies.
D-values can be calculated by using one of two methods, survivor curve2 or fraction negative3 analysis. A survivor curve plots the surviving microorganisms against a critical process parameter, usually time. The curve provides an important graphic representation of the kinetics of the microbicidal process. Fraction negative analysis, sometimes referred to as “endpoint experiments”, focuses on the quantal zone (the area where exposures to replicate units result in both positive and negative units when cultured for growth).
In general, a survivor curve establishes the resistance for surviving populations greater than 5 x 101 whereas the fraction negative method establishes the resistance for surviving populations below 5 x 100.
There are two advantages to the survivor curve method. One is that it can demonstrate a log linear regression curve when performing product testing, which can be extrapolated to demonstrate the calculated sterility assurance level (SAL). The other is that the product can be removed through filtration so as to eliminate any potential product inhibition on the injured spores. Mesa Labs’ Contract Studies Laboratory has years of experience performing these studies on a wide variety of pharmaceutical products for Research & Development and commercial manufacturers.
To have a study performed the manufacturer will need to supply approximately 100 ml of product and a slant containing the bioburden isolate. Mesa Labs can supply spores (traceable to a recognized culture collection) if a bioburden study is not desired. A typical study consists of 1) inoculating the product with the test organism, 2) filling and sealing the spiked product into very small glass ampoules, 3) performing graded exposures on the ampoules in a resistometer, and 4) assaying the ampoules for surviving spores. The data collected from these exposures is used to calculate a D-value. Please contact Mesa Laboratories, Inc. for a detailed protocol.
These D-values can be used to calculate an appropriate SAL for the sterilization process. Mesa Labs can also provide a convenient BI challenge, which complements these studies for routine monitoring of the sterilization process.
REFERENCE
-
ISO 18472:2018 Sterilization of health care products - Biological and chemical indicators - Test equipment
-
ISO 11138-1:2017 Sterilization of health care products – Biological indicators – Part 1: General Requirements Annex C
-
ISO 11138-1:2017 Sterilization of health care products – Biological indicators – Part 1: General Requirements Annex D