Steam Sterilization of Liquid Filled Containers
By Garrett Krushefski and updated by Mesa staff
A long time user of our EZTest® steam biological indicators (BI) recently began to hydrate and sterilize their own growth media in-house. They had been purchasing prepared media from a supplier but initiated this change as a cost savings measure. The instructions on the bucket of powdered media read, “Autoclave at 121°C for 15 minutes.” The customer prepared three batches of media to perform their sterilization cycle development and validation exercises and the following results were obtained. All BIs were growth-negative when observed at the conclusion of the 24-hour incubation period; however, the bottles of media had rampant growth in them after three days of storage at room temperature.
At Mesa Laboratories we are fond of the statement “Spores Don’t Lie”. Did our customer just prove us wrong? Did the spores in fact tell us a lie? As is typical for situations such as these, assessment of the problem starts with questions. The following is the list of questions I posed to the customer and their responses. Review the following and see if you can determine what would cause the spores in the BI to be killed yet the media is clearly not sterile.
Q1: Describe the load: how many bottles of media are in the autoclave and what is the fill volume in each bottle?
A1: A total of thirty bottles are in the load; ten are 500ml bottles with 400ml media, ten are 1,000ml bottles with 900ml media, and ten are 2,000ml bottles with 1800ml media.
Q2: What is the cycle time and temperature?
A2: We set the autoclave according to the instructions provided on the bucket of powdered media...121°C for 15 minutes.
Q3: What is the lot number of the BIs that were used and describe the position of the BIs in the load?
A3: BI lot number is S-407. BIs were placed in the cold spot...front of the autoclave just above the drain line.
Now let us consider the autoclaving instructions provided on the bucket of media which read, “Autoclave at 121°C for 15 minutes.” This statement might be accurate if we specify that this condition (121°C for 15 minutes) is referring to the temperature of the media itself and not to the chamber conditions. I believe a more accurate statement to guide the user would be either:
“Achieve a media temperature of 121°C and hold for 15 minutes” or perhaps, “Sterilize media until a lethality of 15 F0 is achieved as indicated by penetration thermocouples.”
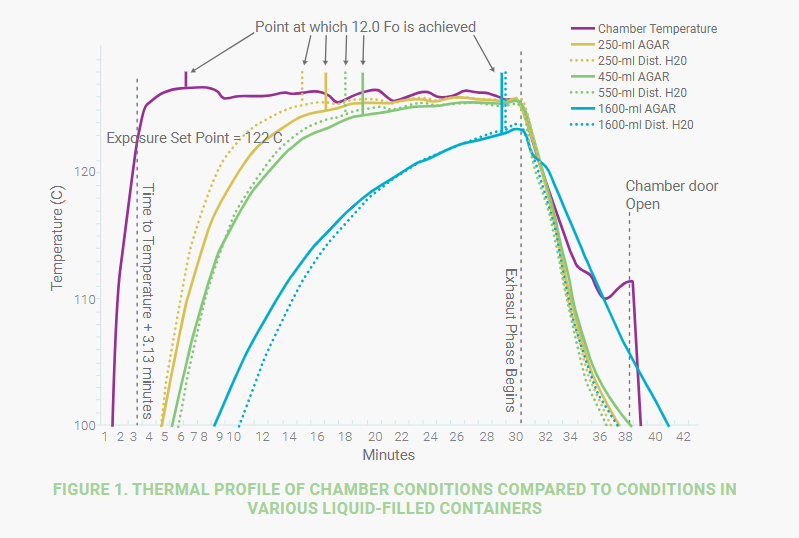
My final concern is the array of volumes the customer tried to process in the same load. Again, refer to Figure 1 and notice the vast difference in sterilization time needed for the various fill volumes. A lethality of 12 F0 was achieved at ~16.5 minutes for the 250ml agar sample whereas ~29 minutes were required to achieve the same lethal delivery to the 1,600ml agar sample. Mixing volumes is acceptable if the difference in volume is not substantial. However, if our customer develops a cycle that is sufficient to sterilize the 400ml mass, the 1,800ml mass will likely not be sterilized. If a longer cycle is developed such that the 1,800ml mass is sterilized, the 400ml mass will risk being “over-baked.” A better approach is to keep liquid fill volumes similar and develop two (or more) distinct sterilization cycles; a shorter cycle that will render small volumes sterile and a longer cycle that can be used when sterilizing larger volumes.
To review, the problems were fixed, and the customer was able to properly validate their media sterilization cycles by implementing the following:
1. Keep the liquid fill volumes similar so that the lag- times-to-temperature will also be similar.
2. Do not trust everything you read. The media instructions directed them to a 15-minute cycle time. Through proper cycle validation exercises, the customer determined that a 40-minute cycle time was needed to deliver 15-minutes of equivalent lethality to their 1,800ml fill volume load.
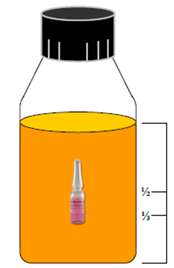
3. Correct BI selection and placement. Sterilization of liquid-filled containers requires use of a liquid- submersible BI. Correct placement of that BI is suspended in the geometric center of the liquid mass, approximately 1/3 to 1/2 of the liquid height off the bottom of the container (Figure 2).