VOL 5, ISSUE 4
Glassine Packaged Vs. Naked Carrier D-values
By Garrett Krushefski, Scientific & Technical Services Manager
When you go into a restaurant for breakfast your server may ask you “How do you want your eggs?” Your choices are to have them cooked in the shell (soft-boiled or hardboiled) or out of the shell (poached, sunny side up, over easy, over hard, or scrambled). So what does this have to do with D-values? The egg shell is analogous to the glassine envelope of the biological indicator (BI). If the egg is cooked in or out of its shell, the time to achieve the desired result will be different. The same is true for the inoculated spore strip if it is removed from the glassine envelope and processed naked.
The BI is a system that includes:
- the spores,
- the carrier material, and
- the primary packaging.
We occasionally receive requests from our users to perform a D-value on the naked spore strip, removed from the glassine envelope. We don’t recommend this practice. It should be understood that spores do not have an intrinsic D-value. With today’s technology there is no way to test spores suspended in space. The spores must be placed in or on a carrier as a necessary convenience of exposing and recovering them to determine the lethal effects delivered by the process. The carrier has an influence on the observed resistance of the bacterial spore. Any packaging that is placed around the carrier will also impact the observed D-values. ISO 14161:2000, paragraph 5.2 reads, “The strain, the production method, the suspension fluid, the carrier, and packaging materials all affect the resistance characteristics of the finished product.”
Dr. Irving Pflug discusses a phenomena which is now commonly referred to as “the envelope effect” on the carrier in his book “Microbiology and Engineering of Sterilization Processes”, thirteenth edition, 2008. Dr. Pflug emphasizes that the spores plus a carrier plus a glassine envelope constitute a system. It is the system that is evaluated when a BI is tested.
Pflug states, “With the arrival of the era of validation, about 1975, to measure the localized effort of the sterilization process, technologists, on occasion, removed the spore strip from the envelope and placed the spore strip (“naked”) in or on a product.” The performance observed was not always that which was expected. Pflug observed that when the spore strip was removed from the envelope and placed into a flask of TSB medium, the exhibited D-value was different from the spore strip in its glassine envelope exposed to the same process. Pflug (2008) reported the following D121°C-value results:
- Strip in envelope - 1.45 minutes
Strip in 10 ml TSB & BCP - 3.94 minutes - Strip in envelope - 0.82 minutes
Strip in 10 ml TSB & BCP - 1.85 minutes
Pflug (2008) also discussed another experiment in which spores from suspension were tested in three different configurations. They included spore strips in glassine envelopes heated in steam, spore strips deposited in and heated in liquid and spores directly inoculated into and heated in liquid.
| Strips in Envelope | Naked Strips in Liquid | Spores in Liquid | |
| D121 | 1.39 minutes | 2.50 minutes | 4.30 minutes |
Mesa has performed experiments using glassine-packaged and naked strips directly exposed in steam and ethylene oxide (EO) Biological Indicator Evaluator Resistometers (BIER). The naked strips exhibited a higher D-value than that observed in the envelope when exposed to steam. The opposite trend was observed for EO testing.
| Strips in Envelope | Naked Strips | |
| D121 Steam | 2.2 minutes | 3.1 minutes |
| DEO | 4.1 minutes | 0.3 minutes |
Mosley, Gillis and Whitbourne (2002) presented DEO values for naked spore carriers embedded into medical devices
| Device | DEO (minutes) |
| Canula | 5.29 |
| Unassembled bone harvesting device | <6.19 |
| Injectable polymer system | 4.09 |
| Rotor blade | 4.16 |
| Suture anchor | 4.00 |
This data clearly indicates that each specific configuration which includes spores plus carrier plus package is a unique system and will exhibit unique observed resistance performance. The resistance of an embedded naked spore strip in a medical device does not compare to, nor have any relevance to the resistance value that would prevail for the naked strip (or the glassine packaged strip) exposed in a BIER. The device carrying the naked spore strip becomes part of its own unique system. This system will have its own unique resistance. Addressing this subject, ISO 14161:2000, paragraph 7.2 states, “Placement of the biological indicator either within the product or within the load is likely to alter its apparent resistance characteristics in comparison to the resistance noted on the labeling of the biological indicator.”
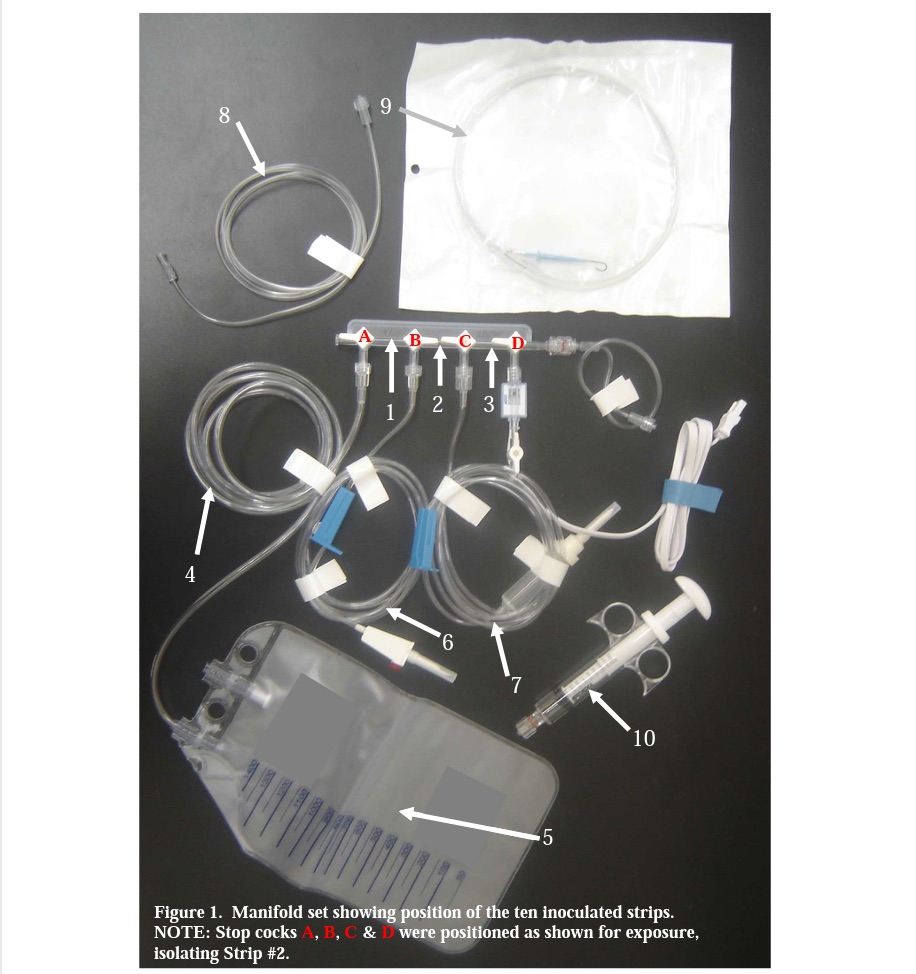
Still not convinced? Considering the following study in which naked spore strips inoculated with 2.9 X 102 Bacillus atrophaeus spores per strip were inserted into the manifold set at the locations identified in Figure 1. When tested in a BIER, the naked strip D-value was 0.4 minutes; the glassine packaged D-value was 3.2 minutes.
The strips at locations 9 & 10 were killed in a 20 minute exposure (the shortest time tested), thus indicating a D-value of ≤ 5.8 minutes. The strips at locations 1, 5, 6 & 7 required an 80 minute exposure for complete kill, thus indicating a D-value of ~ 23.1 minutes. The strips at locations 3 & 4 required a 160 minute exposure for inactivation, indicating a D-value of ~ 46.2 minutes. The strip at location 8 required a 320 minute exposure for inactivation, indicating a D-value of ~ 92.4 minutes. The strip at location 2 was always positive for growth, even in the longest test exposure of 320 minutes, which suggests a D-value in excess of 92.4 minutes! (What is more likely is that no sterilizing agent was reaching this location, by virtue of the closed stop cocks, and the spores on the strips would survive no matter what exposure time was selected.)
Recall that the D-value for the naked test strips was 0.4 minutes and the glassine-packaged D-value was 3.2 minutes. Which is more relevant to the customers intended use in the angiographic set? NEITHER!
If neither D-value has relevance, why test D-value at all? In such circumstances, the labeled D-value on the Certificate of Analysis can only be used as a bench-mark means for comparing lot-to-lot differences in future product received. Mesa selects to test and report the glassine packaged D-value for several reasons:
- Testing the glassine packaged strips is less susceptible to post exposure contamination, which would lead to false positives in the fraction negative data set.
- As a result of the aforementioned, the resulting D-value will be more accurate and reproducible which will facilitate future lot-to-lot comparison of newly received strips.
- Assessment in glassine is easier and less labor intensive; both of which help to keep production costs down which in turn, keeps the price of product affordable.
Mesa provides its customers with quality products at affordable prices. In this case, the assessment in glassine provides the most accurate and reproducible “bench-mark” resistance value in a situation where neither value (glassine or naked assessed) is applicable to the sterilization cycle.
As a user you need to have a well controlled reference system to evaluate spore resistance performance. The glassine envelope and paper carrier has served as a reference system for decades. Resistance testing the naked carrier in a BIER will provide a D-value that has no relevance to the performance when embedded into a device.