VOL 8, ISSUE 1
BI Label Claim Verification Testing by Third Party, Part I
By Kurt McCauley
Introduction
This Spore News is the first in a two part series on the topic of third party testing of biological indicators (BIs). Third party testing is, not surprisingly, often a topic of discussion amongst BI users, manufacturers and third party labs.
Purchasers of BIs have established methods to satisfy themselves that the BIs they purchased are acceptable from a quality standpoint. These methods are not consistent amongst BI users. Many users perform audits of the manufacturer and/ or test the BIs in-house. Others will send their BIs to an outside laboratory to be tested (i.e. a third party test).
The topic of this first paper will be to look at third party requirements as stated in the standards and to provide some statistics on past studies performed at Mesa. The second paper will expand on the discussion and provide some suggestions on how to proceed with future testing.
Generally stated, the testing activities performed on BIs are as follow:
First party testing >> Activities performed by the manufacturer. Testing is generally comprehensive and performed according to recognized standards. The data include population, resistance, purity, etc., and are reported on a Certificate of Analysis for each lot of BIs.
Second party testing >> Activities performed by the user. Users develop internal specification which can vary from minimal or no testing to complete verification of the certified data.
Third party testing >> Activities performed by a lab independent of the above mentioned parties. This testing can vary from minimal to complete verification of the certified data. Mesa is in the unique situation of being involved with both first party testing (as a BI manufacturer) and third party testing (on BIs sent to us from the field).
Additionally, we perform second party testing on BIs used in special studies. The first party testing is performed by the Production Laboratory and the second and third party testing is performed by the Contract Studies Laboratory.
History
A retrospective analysis of the data generated was performed. There were exactly 100 studies performed in that time period. The studies were performed upon request for 21 unique BI users and using product from eight unique BI manufacturers. Most of the BIs were commercially prepared, but a few were custom made biological indicators.
The BI configurations included spore strips (56 studies), self-contained (3 studies), liquid submersible ampoule (9 studies), suspension (19 studies), thread (9 studies) and wire (4 studies). Testing ranged from the basic (purity and population tests) to more all encompassing tests including D-value and survival/kill testing. Population verification was the test most commonly performed followed by D-value studies, survival/kill studies and finally combined D-value-survival/ kill studies. In both the population verification and the survival/kill studies, over 90% of the label claims were confirmed. In the D-value verification studies, approximately 70% of the label claims were confirmed. See Table 1 for a complete summary.
Purity and identification of test organism are not a common request and will not be discussed in any great detail. BI manufacturers generally identify the organism biochemically, genetically or using commercially prepared automated systems and will readily supply this information to the user upon request. Caution should be taken by second and third party labs using certain systems not well suited for identifying the type of organisms typically used in BIs.
Table 1. Summary of the types and number of third party tests performed at Mesa from 2005 through 2010.
| STUDY TYPE | POPULATION ONLY | D-VALUE* | BOTH D-VALUE* AND SURVIVAL & KILL |
| Number of studies performed | 45 | 36 | 4 |
| % of studies confirming certified claims | 91.1% | 69.4% | 75.0% |
*Population verifications are a component of D-value studies
Note: The acceptance criteria used in these studies was based on those stated in the standards. We will discuss this in greater detail in Part II of this Spore News.
ISO 11138-7 (formerly ISO 14161) Standard
ISO 11138-7, the guidance document for BI users, states that users “should have a system in place to provide assurance that the biological indicators obtained consistently meet the specified characteristics.” This is accomplished by obtaining “information from the manufacturer covering the performance characteristics of the lot of biological indicators…” (i.e. a Certificate of Analysis) or by performing “various degrees of testing on each lot of biological indicators...”
Once a user “has established a high level of confidence in the supplier through experience and/or testing, the testing performed by the user may be minimal.” If the user deems testing necessary, then the performance of a population assay and survival-kill resistance testing should be considered. If the BI manufacturer produces product according to ISO 11138, (or other detailed standard specifications), then “testing of the resistance characteristics by the user is considered unnecessary.”
USP
Section <1229.5> (formerly <1035> of the USP Biological Indicators for Sterilization/General Information) discusses “User’s Responsibilities”. Much of these recommendations are much the same as those stated in ISO. Summarizing a few points from USP: The BI user should,
- Establish the BI suitability for use in a specific sterilization process
- Obtain a “Certificate of Performance” on each lot.
- Verify microbial count.
- Observe and note manufacturer’s comments relative to D-value, storage conditions, expiration dating, and stability (users may consider conducting a D-value assessment).
Discussion
As the standards imply, it is critical for a BI user to trust the performance of the BIs in hand. The best method to gain confidence in the BI is to gain confidence in the BI manufacturer. The least costly way to do this is to perform periodic quality audits of the BI manufacturer. The performance of the BI should be trusted if the BI manufacturer can demonstrate that it: 1) is a quality operation; 2) possesses the specialized equipment necessary to perform such tests; 3) employs knowledgeable and trained personnel; and 4) manufactures BIs according to recognized standards.
Once demonstrated, it is acceptable for the user to accept the data presented on the Certificate of Analysis with confidence. The population assay is one relatively simple test the user should consider performing when accepting a lot of BIs. Because the BI units must travel from the manufacturer to the user, this test will ensure that the BIs arrived in good working condition.
Performing quality audits are costly, but not as costly as performing extensive testing on each lot of BIs. Additionally, many lots of BIs will be rejected by second and third party testing, not because of a BI defect, but because of differences in test equipment and techniques. Many papers have been written on this topic and we will discuss this further in Part II of this Spore News.
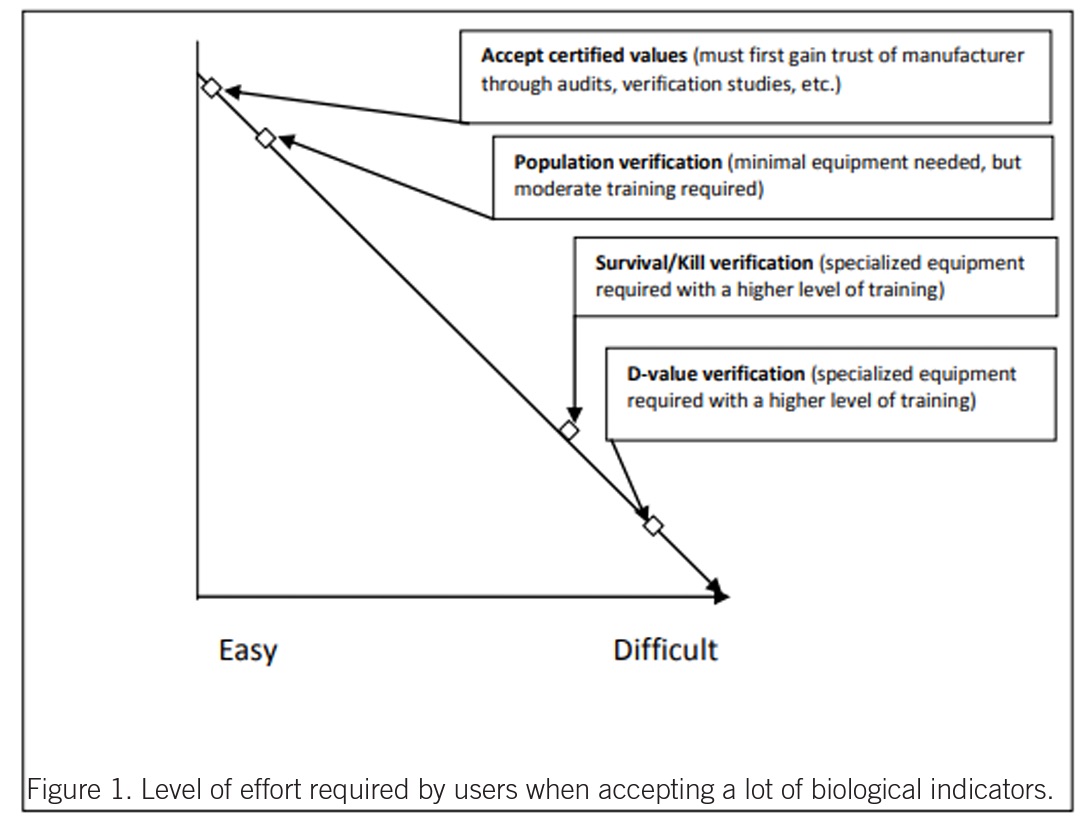
BI users must determine internally the level of testing to be performed on each lot of BIs received. The testing can be done in-house or by a third party. Figure 1 is a simple diagram that illustrates the effort level required for each type of verification study. In the second paper on the third party testing topic, we intend to discuss acceptance criteria, methods of resistance determination (manufacturer vs. user), resistometer types and performance, and BI types.
About the Author
Kurt McCauley has a B.S. in Microbiology and is a Product Development Engineer in the Sterilization and Disinfection Control Division of Mesa Laboratories. He began work at Mesa in 1995 and has been involved with all aspects of biological indictor production and development. Mr. McCauley currently serves as Co-chair for AAMI Working Group 91 (Resistometers), and is an active member of both AAMI/ ISO Working Groups 4 (biological indicators) and 6 (chemical indicators).