VOL 1, ISSUE 4
The Importance of 16S rRNA Bacterial Spore Identification
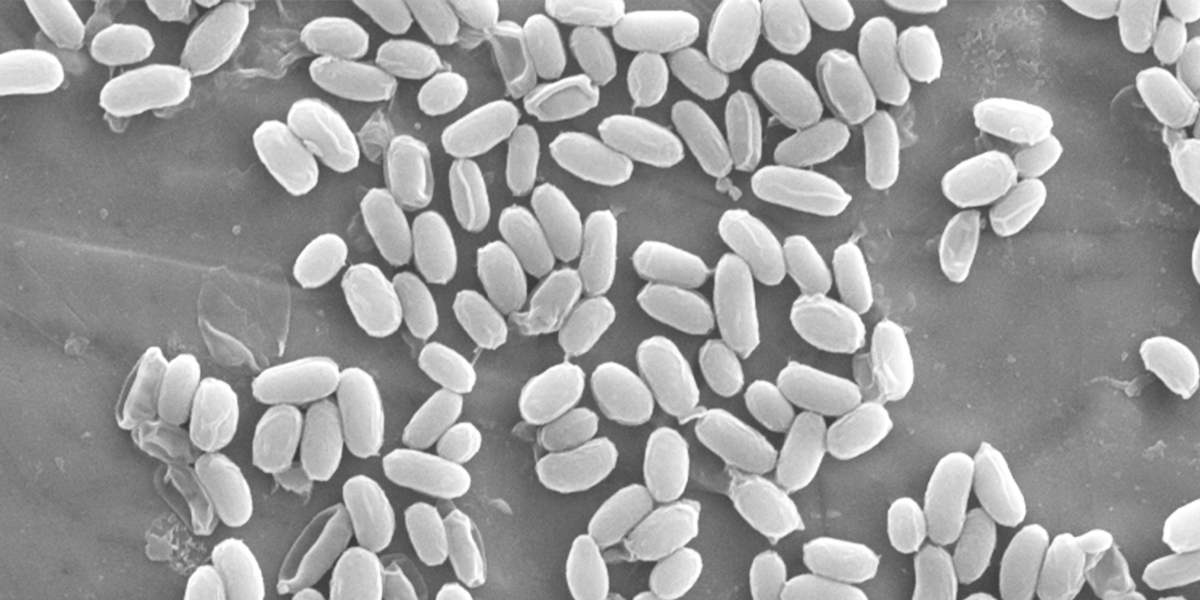
By Kellie Matzinger, Spore Lab Supervisor
The 16S rRNA genetic analysis of spore crops is an essential element in SGM’s Quality Control program. The need for an accurate and precise method for microbial identification should be a priority in any microbiology laboratory. Identifying the causative agent of an infection in a clinical laboratory, confirming the identity of a microorganism in a research laboratory, identifying environmental isolates which may impact the sterilization process of a medical product or confirming the identity of the biological indicator spore challenge used for your BI’s are some of the reasons to perform a 16S rRNA analysis.
Genetic information is conserved throughout the microorganism’s life cycle. It is independent of stage of growth or even viability and is currently the most reliable resource for bacterial identification. One widely accepted genetic determination uses the 16S rRNA. This analysis uses the RNA type found in the ribosomes of all self-replicating cells. The genetic fingerprint or gene sequence is retrieved using a primer that targets a specific gene sequence which captures a 500 base sequence sample. This data is compared to the same 500 base sequence database in such genetic libraries as MicroSeq®, Genbank or Ribosomal Database Project (RDP).
This comparison results in a “percent difference” from the library database and identifies the specific base positions that are different. If the percent difference to the closest library database match is under 1%, the sample is guaranteed to the species. If the differences are greater than 1% and less than 10%, it is guaranteed to the genus level. If greater than a 1% difference is seen, the 500 base sequence would then be compared to
other database libraries to determine if a closer match exists.
Examples of these test results appear in Table 1. Sample KAM1 was identified as Bacillus stearothermophilus (now referred to as Geobacillus stearothermophilus) with a 0.46% percent difference when the 16S rRNA 500 base sequence was aligned with the MicroSeq® sequence. This is a difference in 4 bases out of the 500 bases in the sequences.
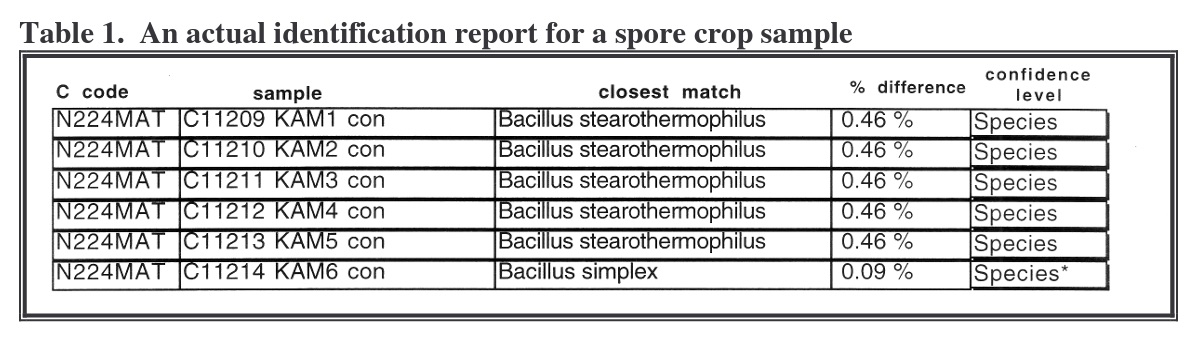
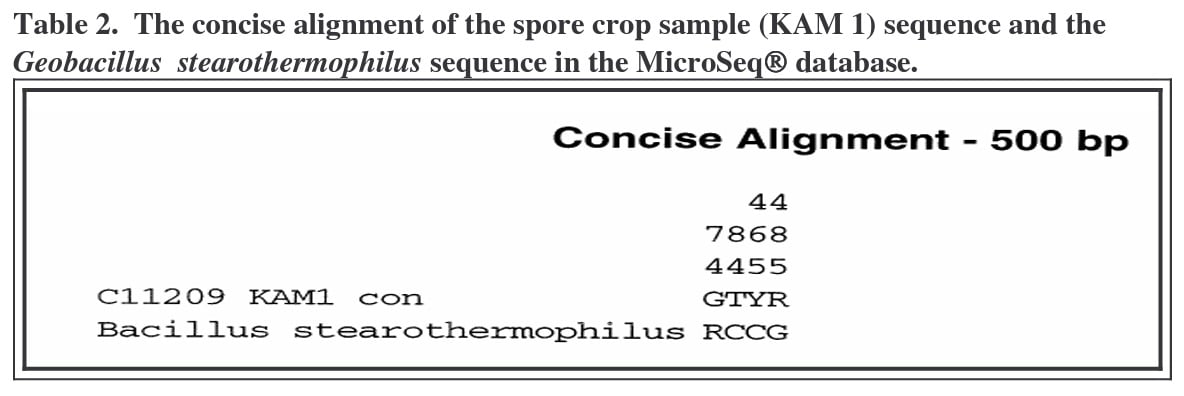
The differences are noted in Table 2. This illustrates positions that differ between KAM 1 sample and the first match in the database. The position of the mismatch is read vertically from top to bottom and the sequences are read horizontally from left to right. The differences occurred at base number 74, where the sample sequence contains a “G” and the database sequence contains a “R”; at base number 84, the sample contains a “T”
and the database sequence contains a “C”; at base number 465, where the spore crop sample contains a “Y” and the database sample contains a “C”; and at base number 485, where the spore crop sample contains an “R” and the database sample contains a “G”. Differences that occur at the end of the sequence may be due to promoter attachment anomalies. Base differences in the interior of the sequence are more likely to be real differences. In this case, all differences were near both sample ends.
At SGM the results obtained from 16S rRNA analysis are not only valuable for species confirmation, but these results are used to verify genetic consistency from one spore crop to another. SGM believes that the resistance performance of bacterial spores directly correlates with genetic information found within the spore. It can be demonstrated that spore crops produced at SGM have no observable genetic variability, as illustrated in Table 1. Samples KAM 1 through KAM 5 are five separate spore crops of Geobacillus stearothermophilus, each cultivated at different times over a six month period, yet each have the same percent difference and base differences. This is one of the many controls that enable SGM to supply consistent biological indicator products.
Regulatory requirements established by USP, ISO, and EN require manufacturers of biological indicators to support traceability to recognized culture collections. These regulations define which microorganism may be used in biological indicators for monitoring the sterilization process. Proof of conformance to these regulations is the basis for good manufacturing practices (GMP’s).
Instrumentation used for identifying microorganisms requires an extensive database. The database has typically been developed for mesophiles or environmental isolates. Only recently have the manufacturers of this instrumentation begun to include sporeformers and thermophiles.
Many laboratories prefer phenotypic methods such as biochemical tests, or analysis of fatty acids to identify microorganisms. Some phenotypic characteristics are carried by plasmids and the loss or addition of plasmids can result in a gain or loss of phenotypic traits. One such example is B. atrophaeus (ATCC No. 9372). The most distinguishing phenotypic characteristics of this species is its ability to form an orange pigment when grown on glucose supplemented agar, and a black pigment when grown on a tyrosine supplemented agar. For this reason it was initially named Bacillus subtilis var niger strain globigii. Cultures recently purchased however, have lost these defining characteristics. The colonies of B. atrophaeus now appear white on both glucose and tyrosine supplemented agar plates. However, genetic identification of these cultures confirms the species identification.
The genetic techniques using the 16S rRNA analysis prove to be the most reliable source for microbial identification. SGM’s goal is to provide customers with the most consistent biological indicator products possible. Every spore crop used for the production of SGM biological indicators is confirmed using this analysis. SGM Biotech’s laboratory cultivates many different species and strains of bacterial spores for use in biological indicators. Common sporeformers include Geobacillus stearothermophilus ATCC No. 7953 and ATCC No. 12980 used for monitoring steam and hydrogen peroxide vapor sterlization applications; Bacillus atrophaeus ATCC No. 9372 for ethylene oxide and dry heat sterilization applications; Bacillus pumilus ATCC No. 27142 for radiation sterilization; Bacillus smithii (formerly Bacillus coagulans) ATCC No. 51232, Bacillus subtilis “5230” ATCC No. 35021, and Clostridium sporogenes for low temperature steam sterilization applications.