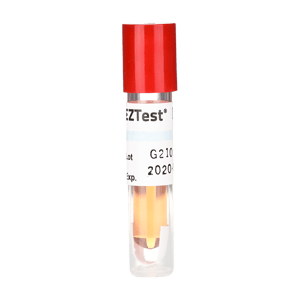
Bowie Dick Simulation Test
-
Bowie Dick Simulation Test
Reusable Process Challenge Device (PCD) designed for routine monitoring of pre-vacuum steam sterilizers.
Product Overview
The Bowie Dick Simulation Test is a breakthrough reusable Process Challenge Device (PCD) that combines both Bowie-Dick and hollow load test requirements in a single system. Designed specifically for pharmaceutical and biotech manufacturing, this type 2 indicator system meets ISO 11140-1 standards and ensures comprehensive steam sterilizer performance verification while dramatically reducing environmental waste.
Key Benefits
- Dual ISO Compliance: Meets both ISO 11140-4 (porous load) and ISO 11140-6 (hollow load) in one integrated device
- Sustainability Leader: Reusable for at least 10,000 cycles, eliminating ~120g of waste per test compared to disposable packs
- Regulatory Ready: Supports daily testing requirements per ISO 17665 and EU GMP Annex 1 (Clause 8.61)
- Cost-Effective: Long-term savings through reusable format and simplified logistics
- Easy Documentation: Self-adhesive indicator strips with graduated color change for permanent archiving
- Space Efficient: Compact design reduces storage requirements by up to 99%
Regional Availability - Not currently available in the United States.
Technical Specifications
- Standards Compliance: ISO 11140-1 (type 2), ISO 11140-4, ISO 11140-6, ISO 17665, EU GMP Annex 1
- Temperature Range: 121°C (15-30 min) or 132-137°C (1-3.5 min)
- Durability: Minimum 10,000 cycles with proper maintenance
- Construction: Stainless steel tube and capsule, temperature-stable plastic housing (up to 200°C)
- Indicator System: Six-bar graduated chemical indicator with irreversible color change
- Seal Ring Replacement: Every 500-1,000 cycles (included with each 100-unit indicator bag)
How It Works
The PCD contains a white Teflon holder with a folded indicator strip featuring six bars that change from yellow to black when proper steam penetration, temperature, and time parameters are achieved. The device simulates both porous and hollow load challenges, detecting trapped air and steam penetration issues that could compromise sterilization effectiveness.
Ideal For
- Pharmaceutical manufacturing facilities with sterile production lines
- Biotech companies subject to EU GMP Annex 1 audits
- Contract Manufacturing Organizations (CMOs) and CDMOs
- Aseptic manufacturing operations
- Facilities transitioning to sustainable sterilization monitoring
Document Downloads
Related Resources
- Video tutorial
Related Articles
Related products
Related services

Contract studies laboratory
Mesa’s independent contract studies laboratory performs certified and compliant third-party chemical and biological indicator testing and verification.
Learn more

Sterilization cycle development
Get expert help for your bioburden. Mesa Labs assists with sterilization cycle development, BI qualification and validation, and routine monitoring support.
Learn more